"NO EXCUSES" Creatine Monohydrate Powder
"NO EXCUSES" Creatine Monohydrate Powder
Couldn't load pickup availability

A must have supplement! Easily mixes with your drink and easy to shotgun!
Product Details & usage
|
Formula Purposes & Benefits |
|
"NO EXCUSES" Creatine Monohydrate Powder is formulated to optimize exercise performance, cognitive function, immune health, and muscle growth. Our top quality creatine contains no fillers, sodium or sugar and is laboratory tested for quality and purity using only 100% pure micronized creatine monohydrate. It works to help those wanting to build muscle mass, increase strength and have the energy needed to complete fitness and bulking objectives.* Our product is synthesized utilizing the latest scientific research and formulated with creatine monohydrate. Our formula is third party independently tested for heavy metals, impurities, made in the USA, GMP certified, and produced in an FDA registered facility. 1% of the supplements on the market can match our world class standards. |
|
Formula Ingredient Deck |
Benefits Of Each Ingredient |
|
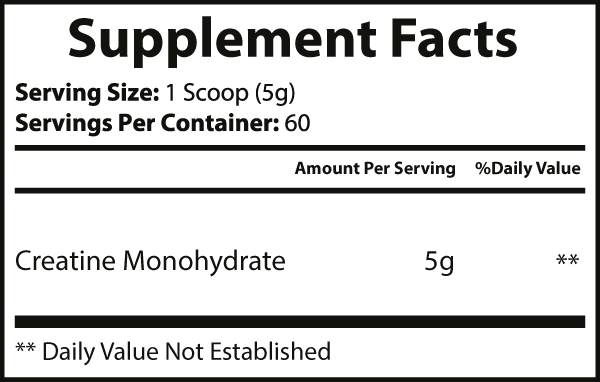
Creatine monohydrate |
|
|
Proper Use of This Supplement |
|
Suggested Use: As a dietary supplement, take one (1) scoop per day with an 8oz glass of water or as directed by your healthcare professional. |
|
Our Formula Vs other formulas on the market. |
|
|
1. Uses third party independently tested ingredients that are made in the USA, GMP certified, and made in an FDA registered facility. |
1. Source cheap ingredients from heavily polluted soils. Even “organic” supplements not third party tested have been removed by FDA due to high levels of heavy metals. |
|
2. 100% creatine monohydrate powder with no fillers. |
2. Uses creatine monohydrate fillers and protein spiking (little amounts of amino acids mixed with fillers). |
Sources:
- Kreider, R. B., Kalman, D. S., Antonio, J., Ziegenfuss, T. N., Wildman, R., Collins, R., … Lopez, H. L. (2017). International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. Journal of the International Society of Sports Nutrition, 14, 18. doi:10.1186/s12970-017-0173-z
- Di Biase, S., Ma, X., Wang, X., Yu, J., Wang, Y. C., Smith, D. J., Zhou, Y., Li, Z., Kim, Y. J., Clarke, N., To, A., & Yang, L. (2019). Creatine uptake regulates CD8 T cell antitumor immunity. The Journal of experimental medicine, 216(12), 2869–2882. https://doi.org/10.1084/jem.20182044
- Kazak, L., & Cohen, P. (2020). Creatine metabolism: energy homeostasis, immunity and cancer biology. Nature reviews. Endocrinology, 16(8), 421–436. https://doi.org/10.1038/s41574-020-0365-5
- Ebrahimi, K., Jourkesh, M., Sadigh-Eteghad, S., Stannard, S. R., Earnest, C. P., Ramsbottom, R., Antonio, J., & Navin, K. H. (2020). Effects of Physical Activity on Brain Energy Biomarkers in Alzheimer's Diseases. Diseases (Basel, Switzerland), 8(2), 18. https://doi.org/10.3390/diseases8020018


